How can graphite and diamond be so different if they are both
Par un écrivain mystérieux
Description

Extreme pressure? Diamonds can take it
Solved Why are the properties of diamond and graphite
Both diamond and graphite are made from carbon. However, diamond is considered the hardest material, while graphite is brittle and slippery. What is this difference, from an atomic bonding view point?

Diamond vs. Graphite: What is the Difference?

The Atomic Difference Between Diamonds and Graphite – Sustainable Nano
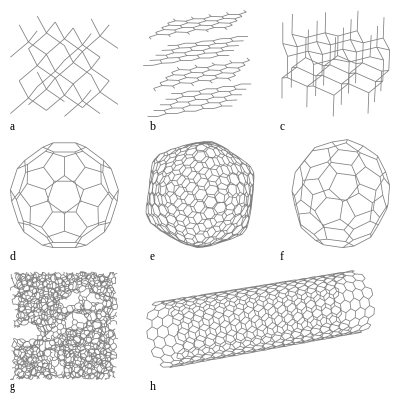
Allotropes of carbon - Wikipedia

Different allotropes of carbon viz Graphite, Diamond, Fullerene, and

What Is an Allotrope? Definition and Examples in Chemistry

Diamond and graphite are

Spontaneity Chemistry for Majors

Why diamond and graphite have different physical properties but same chemical properties? What is the property called? - Quora
How can graphite and diamond be so different if they are both composed of pure carbon?

Scientists Turned Diamond Into Graphite for the First Time

Atomic spacing - Wikipedia
depuis
par adulte (le prix varie selon la taille du groupe)







